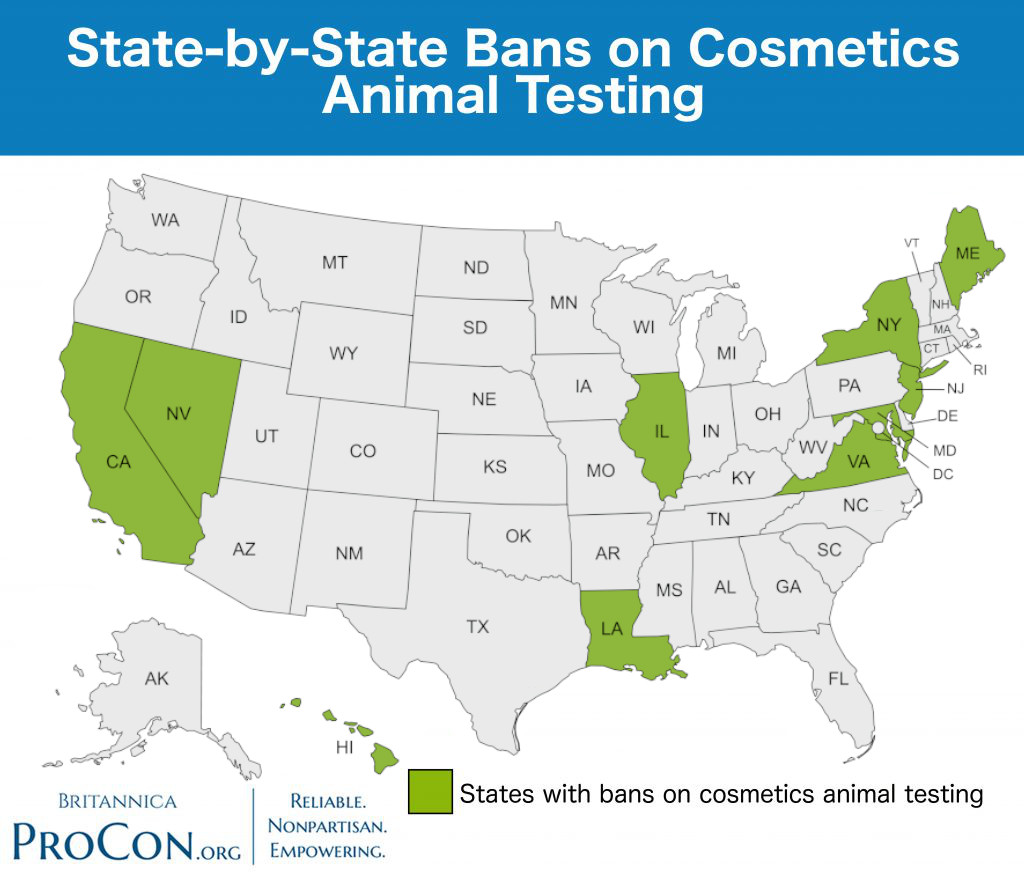
| State | Date Passed | Date Enacted | Bill(s) | Law | |
|---|---|---|---|---|---|
| 1. | California | Sep. 28, 2018 | Jan. 1, 2020 | SB 1249 | California Cruelty-Free Cosmetics Act |
| 2. | Hawaii | July 2, 2021 | Jan. 1, 2022 | SB345 HB1088 | 2022 Hawaii Revised Statutes, Title 19. Health, 321. Department of Health, 321-30.4 Cosmetics; animal testing; prohibition. |
| 3. | Illinois | Aug. 9, 2019 | Aug. 9, 2019 | SB 0241 | Public Act 101-0303 |
| 4. | Louisiana | Jun 18, 2022 | Aug. 1, 2022 | HB 714 | RS 51:§772 - §776 |
| 5. | Maine | June 11, 2021 | Nov. 1, 2021 | LD 1551 HP 1156 | Sec. 1. 10 MRSA c. 233 |
| 6. | Maryland | May 30, 2021 | July 1, 2022 | SB0282 CH0774>/a> | Maryland Health-General Code Ann. § 21-259.3 |
| 7. | Nevada | June 1, 2019 | June 30, 2020 | SB 197 | Chapter 598 of Nevada Revised Statutes |
| 8. | New Jersey | Nov. 8, 2021 | Nov. 8, 2021 | S1726 | P.L. 2021, CHAPTER 272 |
| 9. | New York | Dec.15, 2022 | Jan. 1, 2023 | A5653B S4839B | Chapter 682 |
| 10. | Virginia | Mar. 17, 2021 | Jan. 1, 2022 | HB 2250 SB 1379 | Humane Cosmetics Act |
“Notwithstanding any other law, it is unlawful for a manufacturer to import for profit, sell, or offer for sale in this state, any cosmetic, if the cosmetic was developed or manufactured using an animal test that was conducted or contracted by the manufacturer, or any supplier of the manufacturer, on or after January 1, 2020.”
Exceptions:
“(c) The prohibitions in subdivision (a) do not apply to the following:
(1) An animal test of any cosmetic that is required by a federal or state regulatory authority if all of the following apply:
(A) The ingredient is in wide use and cannot be replaced by another ingredient capable of performing a similar function.
(B) A specific human health problem is substantiated and the need to conduct animal tests is justified and is supported by a detailed research protocol proposed as the basis for the evaluation.
(C) There is not a nonanimal alternative method accepted for the relevant endpoint by the relevant federal or state regulatory authority.
(2) An animal test that was conducted to comply with a requirement of a foreign regulatory authority, if no evidence derived from the test was relied upon to substantiate the safety of the cosmetic sold in California by the manufacturer.
(3) An animal test that was conducted on any product or ingredient subject to the requirements of Chapter V of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 351 et seq.).
(4) An animal test that was conducted for noncosmetic purposes in response to a requirement of a federal, state, or foreign regulatory authority, if no evidence derived from the test was relied upon to substantiate the safety of the cosmetic sold in California by the manufacturer. A manufacturer is not prohibited from reviewing, assessing, or retaining evidence from an animal test conducted pursuant to this paragraph.”
Penalties:
$5,000 fine, with an additional $1,000 per day for each additional day of violation.
“Notwithstanding any other law to the contrary, and except as otherwise provided in this section, it shall be unlawful for a manufacturer to import for profit, sell, or offer for sale in the State any cosmetic for which the manufacturer knew or reasonably should have known that an animal test was conducted or contracted, by or on behalf of the manufacturer or any supplier of the manufacturer, on or after January 1, 2022, in a cruel manner, as identified in section 711-1108.5(1)(a).”
Exceptions:
“This section shall not apply to:
(1) An animal test of a cosmetic that is required by a federal or state regulatory authority if all of the following apply:
(A) The cosmetic or an ingredient in the cosmetic that is being tested is in wide use and cannot be replaced by another cosmetic or ingredient capable of performing a similar function;
(B) A specific human health problem relating to the cosmetic or ingredient is substantiated and the need to conduct animal tests is justified and is supported by a detailed research protocol proposed as the basis for the evaluation of the cosmetic or ingredient; and
(C) There is no non-animal testing method accepted for the relevant purpose by the applicable federal or state regulatory authority
(2) An animal test that was conducted to comply with a requirement of a foreign regulatory authority, if no evidence derived from that test was relied upon to substantiate the safety of a cosmetic sold within the State by the manufacturer;
(3) An animal test that was conducted on any product or ingredient subject to the requirements of subchapter V of the Federal Food, Drug, and Cosmetic Act (21 United States Code 351 et seq.), as amended;
(4) Except as otherwise provided in this subsection, an animal test that was conducted for purposes unrelated to cosmetics pursuant to a requirement of a federal, state, or foreign regulatory agency; provided that no evidence derived from the testing was relied upon to substantiate the safety of a cosmetic sold within this State by the manufacturer; provided further that if evidence from such testing was relied upon for that purpose, the prohibition in paragraph (1) does not apply if:
(A) Documentary evidence exists of the intent of the test that was unrelated to cosmetics; and
(B) The ingredient that was the subject of the testing has been used for purposes unrelated to cosmetics for not less than twelve months prior to the reliance;
(5) A cosmetic if the cosmetic in its final form was tested on animals before January 1, 2022, even if the cosmetic is manufactured on or after that date;
(6) An ingredient in a cosmetic if the ingredient was sold in this State and tested on animals before January 1, 2022, even if the ingredient is manufactured on or after that date; or
(7) A manufacturer reviewing, assessing, or retaining evidence from animal testing as defined in this section.”
Penalties:
$5,000 fine, with an additional $1,000 per day for each additional day of violation.
“Notwithstanding any other law, it is unlawful for a manufacturer to import for profit, sell, or offer for sale in this State any cosmetic, if the cosmetic was developed or manufactured using an animal test that was conducted or contracted by the manufacturer, or any supplier of the manufacturer, on or after January 1, 2020.”
Exceptions:
“(c) The prohibitions in subsection (b) do not apply to the following:
(1) An animal test of any cosmetic that is required by a federal or State regulatory authority, if each of the following apply:
(A) an ingredient is in wide use and cannot be replaced by another ingredient capable of performing a similar function;
(B) a specific human health problem is substantiated and the need to conduct animal tests is justified and supported by a detailed research protocol proposed as the basis for the evaluation; and
(C) there is not a nonanimal alternative method accepted for the relevant endpoint by the relevant federal or State regulatory authority.
(2) An animal test that was conducted to comply with a requirement of a foreign regulatory authority, if no evidence derived from the test was relied upon to substantiate the safety of the cosmetic being sold in Illinois by the manufacturer.
(3) An animal test that was conducted on any product or ingredient subject to the requirements of Subchapter V of the Federal Food, Drug, and Cosmetic Act.
(4) An animal test that was conducted for noncosmetic purposes in response to a requirement of a federal, State, or foreign regulatory authority, if no evidence derived from the test was relied upon to substantiate the safety of the cosmetic sold in Illinois by the manufacturer. A manufacturer is not prohibited from reviewing, assessing, or retaining evidence from an animal test conducted under this paragraph.”
Penalties:
$5,000 fine for the first day of each violation, with an additional $1,000 per day for each additional day of violation.
“Notwithstanding any provision of law to the contrary, it is unlawful for a manufacturer to sell or offer for sale in this state a cosmetic that utilized cosmetic animal testing during the development or manufacture of the cosmetic, if the cosmetic animal testing was conducted by the manufacturer, any supplier of the manufacturer, or any person or business hired or contracted by the manufacturer.”
Exceptions:
“The provisions of this Part shall not apply to the following instances of cosmetic animal testing:
(1) Cosmetic animal testing conducted outside of the United States as required by a foreign regulatory authority, provided that no evidence derived from the testing was relied upon to substantiate the safety of the cosmetic ingredient or cosmetic product being sold by the manufacturer in this state.
(2) Cosmetic animal testing conducted for any cosmetic or cosmetic ingredient subject to regulation under 21 U.S.C. 351 et seq.
(3) Cosmetic animal testing conducted for a cosmetic ingredient intended to be used in a product that is not a cosmetic product and conducted pursuant to a requirement of a federal, state, or foreign regulatory authority, provided that no evidence derived from the testing was relied upon to substantiate the safety of a cosmetic sold in this state by a cosmetics manufacturer, unless all of the following apply:
(a) There is no nonanimal alternative method or strategy recognized by any federal or state agency or the Organisation for Economic Co-operation and Development for the relevant safety endpoints for the cosmetic ingredient or nonfunctional constituent.
(b) There is documented evidence of the noncosmetic intent of the test.
(c) There is a history of use of the ingredient outside of cosmetics at least twelve months prior to reliance.
(4) Cosmetic animal testing requested, required, or conducted by a federal or state regulatory authority if all of the following apply:
(a) There is no nonanimal alternative method or strategy recognized by any federal or state agency or the Organisation for Economic Co-operation and Development for the relevant safety endpoints for the cosmetic ingredient or nonfunctional constituent.
(b) The cosmetic ingredient or nonfunctional constituent poses a risk of causing a specific substantiated human health problem and the need to conduct cosmetic animal testing is justified and supported by a detailed research protocol proposed as the basis for the evaluation of the cosmetic ingredient or nonfunctional constituent.
(c) The cosmetic ingredient or nonfunctional constituent is in wide use and, in the case of a cosmetic ingredient, cannot be replaced by another cosmetic ingredient capable of performing a similar function.”
B. The provisions of this Part shall not apply to any of the following:
(1) A cosmetic in its final form, which was tested on animals before the effective date of this Part, regardless of whether the cosmetic is manufactured on or after the effective date of this Part.
(2) An ingredient in a cosmetic, which was tested on animals before the effective date of this Part, even if the ingredient is manufactured on or after the effective date of this Part.
(3) A cosmetic manufacturer reviewing, assessing, or retaining evidence from a cosmetic animal test.”
Penalties:
A fine up to $1,000 for the first day of each violation, with an additional $500 per day for each additional day of violation.
“Notwithstanding any other provision of law to the contrary, a manufacturer may not sell or offer to sell in the State a cosmetic if the cosmetic was developed or manufactured using cosmetic animal testing that was conducted or contracted for by the manufacturer or any supplier of the manufacturer on or after November 1, 2021.”
Exceptions:
“This section does not apply to:
A. Cosmetic animal testing:
(1) Conducted outside of the United States and in order to comply with a requirement of a foreign regulatory authority as long as no evidence derived from the testing was relied upon to substantiate the safety of the cosmetic ingredient or cosmetic product being sold by the manufacturer in the State;
(2) Conducted for any cosmetic or cosmetic ingredient subject to regulation under Chapter V of the Federal Food, Drug, and Cosmetic Act, 21 United States Code, Section 351;
(3) Conducted for a cosmetic ingredient intended to be used in a product that is not a cosmetic product and conducted pursuant to a requirement of a federal, state or foreign regulatory authority as long as no evidence derived from the testing was relied upon to substantiate the safety of a cosmetic sold in this State by a manufacturer, unless all of the following apply:
(a) There is no nonanimal alternative method or strategy recognized by any federal or state agency or the International Organisation for Economic Cooperation and Development or its successor organization for the relevant safety endpoints for the cosmetic ingredient or nonfunctional constituent;
(b) There is documented evidence of the noncosmetic intent of the test; and
(c) There is a history of use of the ingredient outside of cosmetics at least 12 months prior to the reliance; or
(4) Requested, required or conducted by a federal or state regulatory authority and all of the following apply:
(a) There is no nonanimal alternative method or strategy recognized by any federal or state agency or the International Organisation for Economic Cooperation and Development or its successor organization for the relevant safety endpoints for the cosmetic ingredient or nonfunctional constituent;
(b) The cosmetic ingredient or nonfunctional constituent poses a risk of causing a specific human health problem that is substantiated and the need to conduct cosmetic animal testing is justified and is supported by a detailed research protocol proposed as the basis for the evaluation of the cosmetic ingredient or nonfunctional constituent; and
(c) The cosmetic ingredient or nonfunctional constituent is in wide use and, in the case of a cosmetic ingredient, cannot be replaced by another cosmetic ingredient capable of performing a similar function; [PL 2021, c. 160, §1 (NEW).]
B. A cosmetic if the cosmetic in its final form was tested on animals before November 1, 2021, even if the cosmetic is manufactured on or after that date as long as no new cosmetic animal testing in violation of this section occurred on or after November 1, 2021; [PL 2021, c. 160, §1 (NEW).]
C. A cosmetic ingredient if it was tested on animals before November 1, 2021, even if the ingredient is manufactured on or after that date as long as no new cosmetic animal testing in violation of this section occurred on or after November 1, 2021; or [PL 2021, c. 160, §1 (NEW).]
D. A cosmetic manufacturer reviewing, assessing or retaining evidence from a cosmetic animal test. “
Penalties:
A fine up to $5,000 for the first day of the violation, with an additional fine of up to $1,000 for each additional day of violation.
“(1) Except as provided in subsection (c) of this section, a person may not conduct or contract for animal testing in the development of a cosmetic.
(2) Except as provided in subsection (c) of this section, beginning July 1, 2022, a manufacturer may not sell or offer for sale in the State a cosmetic if the manufacturer knows or reasonably should have known that the final product or any individual component of the final product was developed or manufactured using animal testing that was conducted or contracted by or for the manufacturer or any entity that supplies, directly or through a third party, any ingredient used by a manufacturer in the formulation of a cosmetic on or after January 1, 2022.”
Exceptions:
“(c) The provisions of subsection (b) of this section do not apply to animal testing that is:
(1) Conducted or contracted to comply with a requirement of a federal or state regulatory agency if:
(i) The cosmetic or ingredient in the cosmetic that is tested is in wide use and cannot be replaced by another ingredient that is capable of performing a similar function in the product;
(ii) A specific human health problem relating to the cosmetic or an ingredient in the cosmetic is substantiated and the need to conduct animal testing is justified and supported by a detailed protocol for research that is proposed as the basis for the evaluation of the cosmetic or ingredient in the cosmetic; and
(iii) Animal testing is the only method of testing that is accepted for the relevant purpose by the federal or state regulatory agency;
(2) Conducted or contracted to comply with the requirement of a regulatory agency of a foreign jurisdiction if: (i) No evidence derived from the testing was relied on to substantiate the safety of a cosmetic sold by the manufacturer within the State; and (ii) The testing was not conducted in the State;
(3) Performed on a cosmetic or an ingredient in a cosmetic subject to the requirements of Subchapter V of the Federal Food, Drug, and Cosmetic Act;
(4) Conducted or contracted to comply with a requirement of a federal, state, or foreign regulatory agency for purposes unrelated to cosmetics testing if:
(i) No evidence derived from the testing was relied on to substantiate the safety of a cosmetic sold by the manufacturer within the State; or
(ii) 1. Documentary evidence demonstrates that the intent of the test that was performed was unrelated to cosmetics testing; and 2. The ingredient that was the subject of the testing has been used for purposes unrelated to cosmetics for at least 12 months; or
(5) Performed on:
(i) A cosmetic that, in its final form, was tested on animals before January 1, 2022, whether or not the cosmetic is manufactured on or after January 1, 2022; or
(ii) A cosmetic ingredient that was sold in the State and tested on animals before January 1, 2022, whether or not the ingredient is manufactured on or after January 1, 2022, if any animal testing of the cosmetic ingredient after January 1, 2022, is conducted or relied on in accordance with this section.”
Penalties:
A fine up to $5,000 for the first violation, with an additional fine of up to $1,000 for each additional violation.
“Except as otherwise provided in this section, a manufacturer shall not import for profit, sell or offer for sale in this State any cosmetic for which the manufacturer knew or reasonably should have known that animal testing was conducted or contracted by or on behalf of the manufacturer or any supplier of the manufacturer if the animal testing was conducted on or after January 1, 2020.”
Exceptions:
“2. The prohibition in subsection 1 does not apply to animal testing that is conducted:
(a) To comply with a requirement of a federal or state regulatory agency if:
(1) The cosmetic or ingredient in the cosmetic which is tested is in wide use and cannot be replaced by another ingredient which is capable of performing a similar function;
(2) A specific human health problem relating to the cosmetic or ingredient is substantiated and the need to conduct animal testing is justified and supported by a detailed protocol for research that is proposed as the basis for the evaluation of the cosmetic or ingredient; and
(3) There does not exist a method of testing other than animal testing that is accepted for the relevant purpose by the federal or state regulatory agency.
(b) To comply with a requirement of a regulatory agency of a foreign jurisdiction, if no evidence derived from such testing was relied upon to substantiate the safety of a cosmetic sold within this State by the manufacturer.
(c) On any product or ingredient in the cosmetic subject to the requirements of Subchapter V of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. §§ 351 et seq.
(d) Except as otherwise provided in this paragraph, for purposes unrelated to cosmetics pursuant to a requirement of a federal, state or foreign regulatory agency provided that no evidence derived from such testing was relied upon to substantiate the safety of a cosmetic sold within this State by the manufacturer. If evidence from such testing was relied upon for that purpose, the prohibition in subsection 1 does not apply if:
(1) Documentary evidence exists of the intent of the test which was unrelated to cosmetics; and
(2) The ingredient that was the subject of the testing has been used for purposes unrelated to cosmetics for not less than 12 months before the earliest date of the testing.”
Penalties:
NRS 598.0999 Civil and criminal penalties for violations
“No person or manufacturer shall sell or offer for sale in the State any cosmetic that was developed or manufactured using an animal test, if the test was conducted or contracted by the manufacturer or any supplier of the manufacturer on or after… the effective date” of P.L. 2021, CHAPTER 272 [Nov. 8, 2021].
Exceptions:
c. The prohibitions in subsection b. of this section do not apply to cosmetics developed or manufactured using an animal test if:
(1) The animal test is required by a federal or State regulatory authority and:
(a) the ingredient that requires an animal test is in wide use and cannot be replaced by another ingredient,
(b) a specific human health problem is associated with the ingredient and the need to conduct an animal test on the ingredient is justified and supported by a research protocol, and
(c) there is no non-animal test 1method or strategy1 that is accepted by the relevant federal or State regulatory authority as a means to gather the relevant data;
(2) The animal test is conducted 1outside of the United States to comply with a requirement of a foreign regulatory authority, if no evidence derived from the test is relied upon to substantiate the safety of the cosmetic pursuant to federal or State regulations; or
(3) The animal test is conducted on a product or ingredient subject to the requirements of chapter V of the federal “Food, Drug, and Cosmetic Act,” 21 U.S.C. s.351 et seq. 1; or
(4) The animal test is conducted for non-cosmetic purposes pursuant to a requirement of a federal, State, or foreign regulatory authority. tion of this act, and the court may proceed in the action in a summary manner. No evidence derived from animal testing after the effective date of [P.L. 2021, CHAPTER 272] may be relied upon to establish the safety of a cosmetic pursuant to federal or State regulation unless:
(a) there is no non-animal method or strategy recognized by any federal agency or the Organisation for Economic Co-operation and Development for the relevant safety endpoints for the ingredient;
(b) there is documented evidence of the non-cosmetic intent of the test; and
(c) there is a history of use of the ingredient outside of cosmetics at least one year prior to the reliance on the data.
(d) The prohibitions in subsection b. of this section do not apply to cosmetics that were sold in the State or tested on animals prior to January 1, 2020, even if the cosmetic is manufactured after that date The provisions of this section shall not apply to animal testing conducted on an ingredient or cosmetic if the testing took place prior to January 1, 2021 the effective date of [P.L. 2021, CHAPTER 272].
This section shall not prevent a manufacturer from reviewing, assessing, or retaining data resulting from animal testing.”
Penalties:
A fine up to $1,000 for the first violation. “If the violation is of a continuing nature, each day during which it continues constitutes an additional, separate, and distinct offense.”
“Except as otherwise provided in this section, it shall be unlawful for a manufacturer to import for profit, sell or offer for sale in the state, any cosmetic which the manufacturer knew or reasonably should have known that animal testing was conducted or contracted by or on behalf of the manufacturer or any supplier of the manufacturer if the animal testing was conducted after the effective date of this section.”
Exceptions:
“This section does not apply to animal testing that is conducted:
(A) as a requirement of any federal or state regulatory agency if:
(I) the cosmetic or an ingredient in the cosmetic which is being tested d is in wide use and cannot be replaced by another ingredient which is capable of performing a similar function; and
(Ii) a specific human health problem relating to the cosmetic or ingredient is substantiated and the need to conduct animal testing is justified and supported by a detailed protocol for research that is proposed as the basis for the evaluation of the cosmetic or ingredient; And
(Iii) there does not exist a method of testing other than animal testing that is accepted for the relevant purpose by a federal or state regulatory agency.
(B) as a requirement of any regulatory agency of a foreign jurisdiction, if no evidence derived from such testing was relied upon to substantiate the safety of a cosmetic sold within the state by the manufacturer.
(C) for any product or ingredient in a cosmetic which is subject to the requirements under 21 USC Subchapter V.
(D) for purposes not related to cosmetics as required by any federal, state or foreign regulatory agency, provided that no evidence derived from such testing was relied upon to substantiate the safety of a cosmetic sold within the state by the manufacturer, unless:
(I) documentary evidence exists that the intent of the animal testing was unrelated to cosmetics; and
(Ii) there is a history of the use of the ingredient unrelated to cosmetics for a minimum of twelve months.
4. This section does not apply to a cosmetic:
(A) if in its final form, such cosmetic was tested on animals before the effective date of this section, even if the cosmetic is manufactured on or after such date.
(B) if an ingredient contained in such cosmetic was tested on animals and sold in New York State before the effective date of this section, even if such ingredient is manufactured on or after such date.””
Penalties:
A fine up to $1,000. “If the violation is of a continuing nature, each day during which it continues constitutes an additional, separate, and distinct offense.”
“A. Except as provided in subsection B, no cosmetics manufacturer shall:
1. Conduct or contract for cosmetic animal testing that occurs in the Commonwealth on or after January 1, 2022;
2. Manufacture or import for profit into the Commonwealth any cosmetic or ingredient thereof, if the cosmetics manufacturer knew or reasonably should have known that the cosmetic or any component thereof was developed or manufactured using cosmetic animal testing that was conducted on or after January 1, 2022; or
3. Beginning July 1, 2022, sell or offer for sale within the Commonwealth any cosmetic, if the cosmetics manufacturer knows or reasonably should know that the cosmetic or any component thereof was developed or manufactured using cosmetic animal testing that was conducted on or after January 1, 2022.”
Exceptions:
“B. The prohibitions in subsection A shall not apply to cosmetic animal testing or a cosmetic for which cosmetic animal testing was conducted, if the cosmetic animal testing was conducted:
1. To comply with a requirement of a federal or state regulatory agency and (i) the tested ingredient is in wide use and cannot be replaced by another ingredient capable of performing a similar function; (ii) a specific human health problem related to the cosmetic or ingredient is substantiated that justifies the need to conduct the cosmetic animal testing, and such testing is supported by a detailed research protocol proposed as the basis for the evaluation of the cosmetic or ingredient; and (iii) there does not exist a method of testing other than cosmetic animal testing that is accepted for the relevant purpose by the federal or state regulatory agency;
2. To comply with a requirement of a regulatory agency of a foreign jurisdiction, so long as no evidence derived from such testing was relied upon to substantiate the safety of a cosmetic sold within Virginia by the cosmetics manufacturer;
3. On any cosmetic or cosmetic ingredient subject to the requirements of Subchapter V of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 351 et seq.); or
4. Pursuant to a requirement of a federal, state, or foreign regulatory agency for a purpose unrelated to cosmetics, provided that either no evidence derived from such testing was relied upon to substantiate the safety of the cosmetic or there is (i) documented evidence of a noncosmetic intent of the test and (ii) a history of use of the ingredient outside of cosmetics for at least 12 months prior to such reliance.”
Penalties:
A fine up to $5,000, with an additional $1,000 per day for each additional day of violation.